Which of the following measurements has the greatest precision. A quantity must be divided by multiples of ten when converting from a larger unit to a smaller unit.

Measurement Uncertainty Accuracy And Precision Introductory Chemistry Lecture Lab
This problem has been solved.
. This means its mass lies between 6722 and 6724 grams an uncertainty of 0001 gram. Consider a thermometer having the scale range up to 500ºC. A Accuracy is how far out the measurement is taken while precision is how close a.
Scientists take scientific measurements carefully in. I believe the correct answer from the choices listed above is option C. The more significant figures in the measurement means the more precise the measurement is.
Of the following measurements 100 1000 and 1 10000 has the greatest precision. Which of the following measurements has the greatest precision and why. A measure of how close a series of measurements are to one another.
1 Accuracy refers to how close measurements are to the true value while precision refers to how close measurements are to each other or accuracy is the d View the full answer Transcribed image text. The thermometer has an accuracy of 05 ie. What is the difference between accuracy and precision.
Measurements Complete the following 1. The precise number of choices listed with the question is zero. Which of the following is the SI unit used to measure length.
05 percent of increase or decrease in the value of the instrument is negligible. Which of the following measurements has the greatest precision and why. A kilogram B liter C meter D Kelvin.
A 100 B 1000 C 10000 D 1. Is what you would get using a calculator that has an eight-digit output. A π r2 31415927 12 m 2 45238934 m 2.
But because the radius has only two significant figures it limits the calculated quantity to two significant figures or A 45m 2. See answer 1 Best Answer. The measurement that has the greatest precision is 10000.
Added 7262020 94024 PM This answer has been confirmed as correct and helpful. 10000 has the greatest precision. See the answer See the answer See the answer done loading.
But if the reading is more or less than 05ºC it is considered a high. The quarter weighs about 672 grams with a nominal uncertainty in the measurement of 001 gram. Sets found in the same folder.
Which of the following measurements has the greatest precision. If we weigh the quarter on a more sensitive balance we may find that its mass is 6723 g. Hope this answers the question.
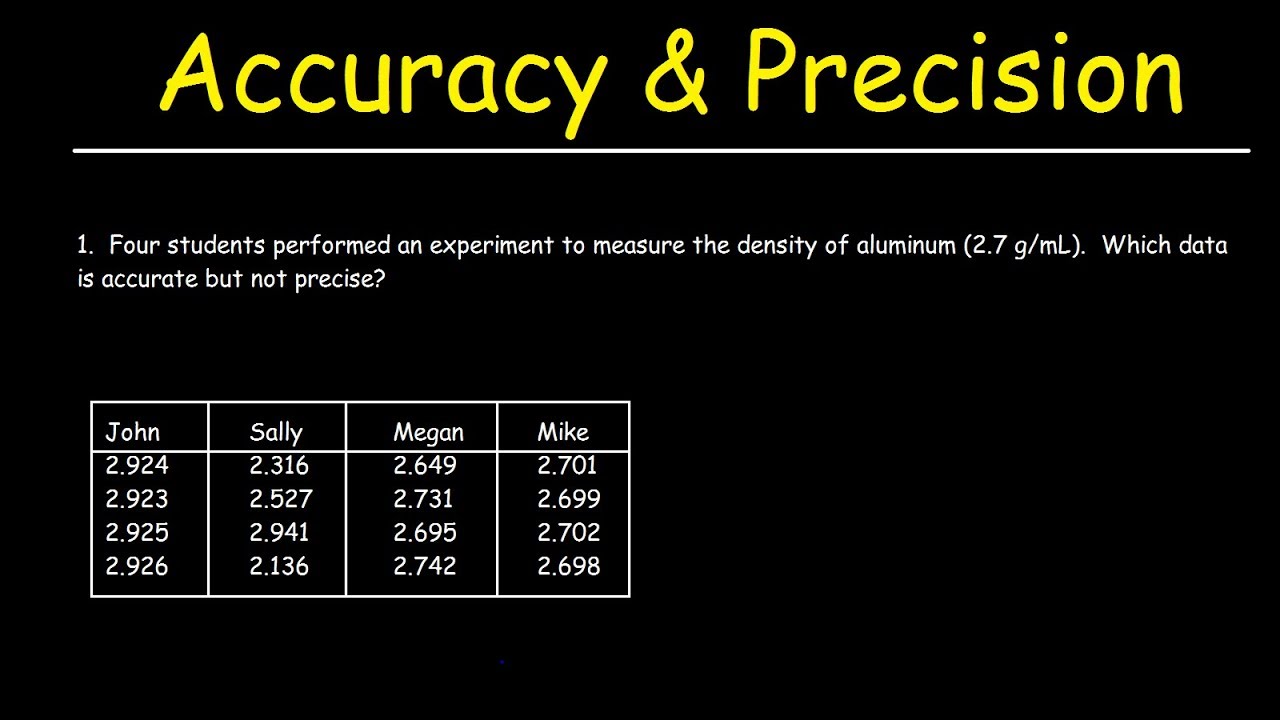
Accuracy And Precision Youtube
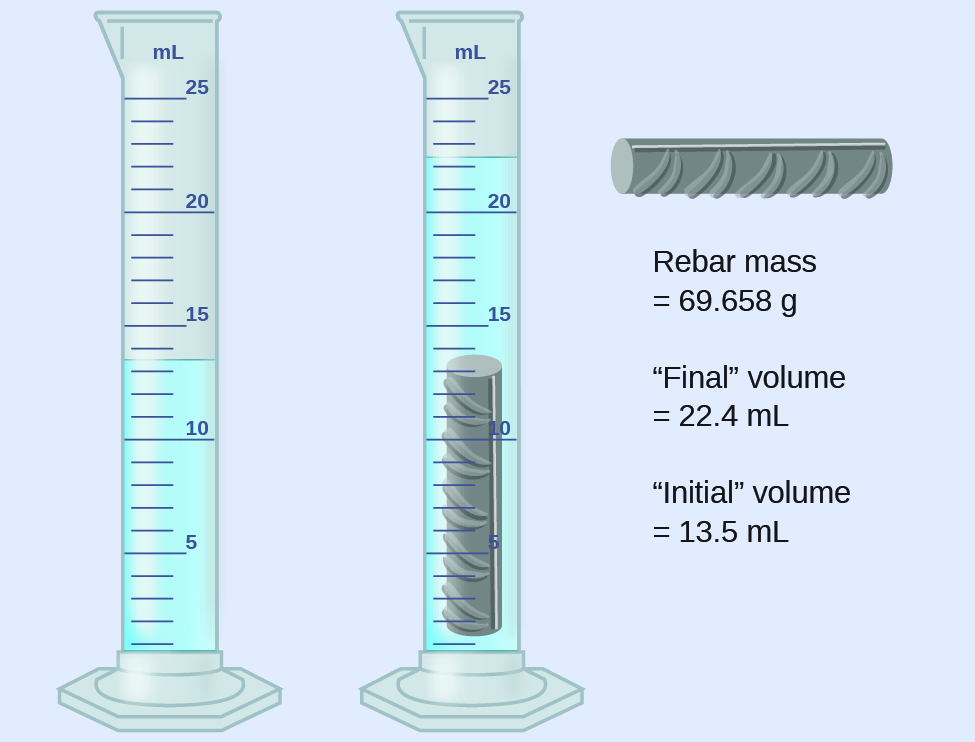
1 5 Measurement Uncertainty Accuracy And Precision Chemistry
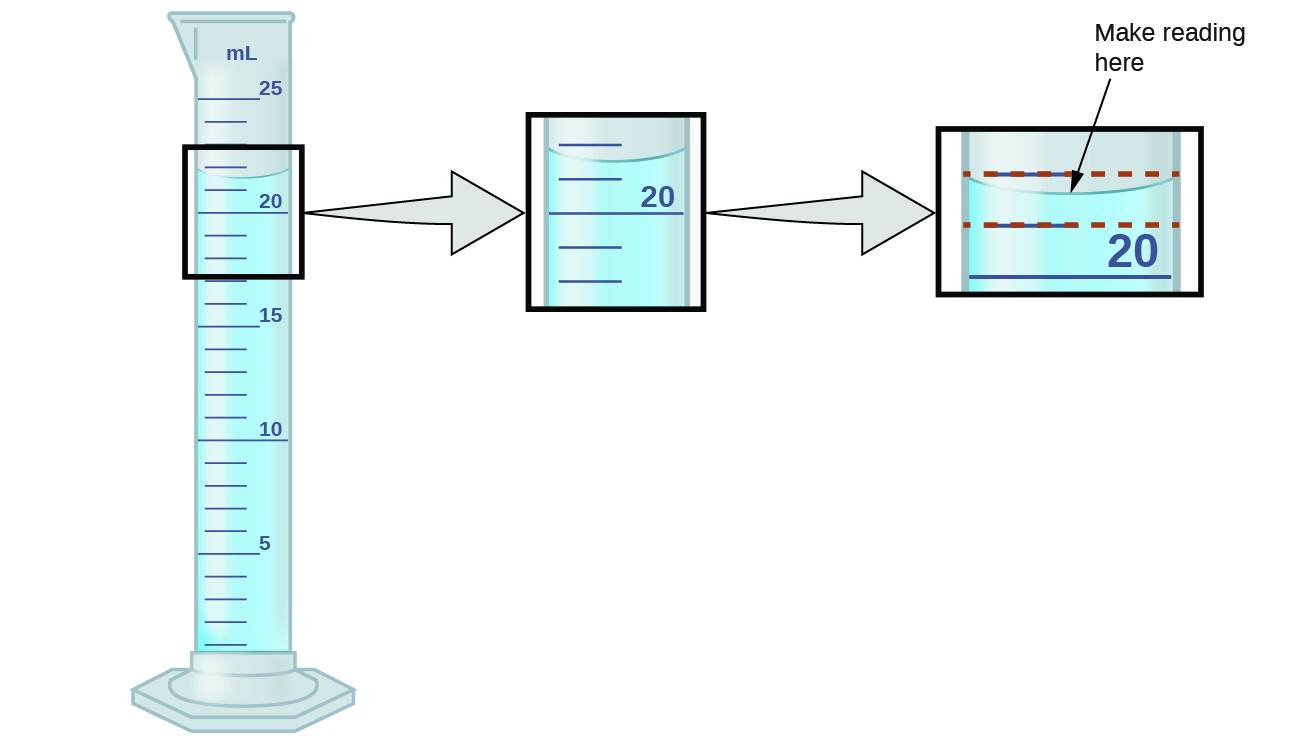
1 5 Measurement Uncertainty Accuracy And Precision Chemistry
0 Comments